Our lives are fundamentally impacted by energy. Understanding the energy composition of various fuels is essential for effective energy usage, whether we’re using it for home heating or car fuel. When it comes to fuels, the terms Higher Calorific Value (HCV) and Lower Calorific Value (LCV) are crucial measures that allow us to assess and contrast the energy content of various fuels.
Each and every fuel has its own energy content in the form of chemical energy. This chemical energy release depends on the inherent properties known as calorific value. Before we use any fuel, we must have information about the calorific value, flash point, fire point, density, specific heat, pour point, etc.
Any fuel that is to be used in any combustion process, first checked by its Calorific Value, namely higher calorific values (HCV) and lower calorific values (LCV). The bomb calorimeter determines these values experimentally. The cost of fuel is also affected by the CV of the fuel. Higher the CV, higher the cost of that fuel.
Table of Contents
What is combustion?
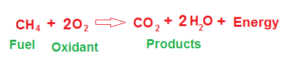
Combustion is defined as a sequence of exothermic reactions/processes in the presence of oxygen/oxidant that at the end give off heat as energy and by-products of combustion products. for example, the combustion of methane with oxygen gives water and carbon dioxide energy.
The calorific Value (CV) of the fuel
Alternately known as Heating Value or Energy Value, CV is defined as the amount of energy liberated by the complete combustion of the unit quantity of fuel.

- Unit: Joule per kg or J/kg
- It’s a characteristic of fuel, based on this number, a fuel is selected for a particular application, and the price is also decided based on the CV.
Some common fuels and its CV depending on the specific composition and source of the fuel.
- Coal:
- Anthracite coal: Approximately 32 to 33 MJ/kg (megajoules per kilogram)
- Bituminous coal: Approximately 24 to 35 MJ/kg
- Sub-bituminous coal: Approximately 17 to 24 MJ/kg
- Lignite coal: Approximately 8 to 17 MJ/kg
- Natural Gas:
- Methane: Approximately 50 to 55 MJ/kg
- Liquefied Petroleum Gas (LPG): Approximately 46 to 50 MJ/kg
- Petroleum Products:
- Gasoline (Petrol): Approximately 44 to 47 MJ/kg
- Diesel: Approximately 42 to 46 MJ/kg
- Jet fuel: Approximately 42 to 47 MJ/kg
- Wood:
- Dry wood: Approximately 15 to 20 MJ/kg (varies with species and moisture content)
- Hydrogen:
- Approximately 120 MJ/kg
- Biofuels:
- Biodiesel: Approximately 37 to 40 MJ/kg
- Ethanol: Approximately 26 to 30 MJ/kg
- Methanol:
- Approximately 19.7 MJ/kg
Types of Calorific Value:
Higher or Gross Calorific Value (HCV) or GCV
If fuel contains hydrogen and if it reacts with oxygen during the combustion process, the product of combustion also contains water vapor or steam. If this water vapor or steam is condensed at the temperature at which fuel and oxygen are supplied (normally room temperature), vapor will be condensed into water form and give off its latent energy (of water). This is additional energy liberated by unit mass fuel. Thus this is called HCV.
HCV = LCV + Latent heat of vaporization of water
Lower or Net Calorific Value (LCV) or NCV
If water vapor as the product of combustion is not allowed to be condensed in water form, the net energy obtained by the complete combustion of a unit quantity of fuel is called lower calorific or net calorific value. For practical purposes, LCV is used in consideration.
In short, LCV and HCV represent the amount of heat released after the complete combustion of fuel without considering the condensate heat and with consideration of condensate heat respectively. One can use a bomb calorimeter to measure the CV of a fuel.
Conclusion
In real-world situations, the surroundings as well as the particular requirements will determine whether to use HCV or LCV. HCV is frequently utilized in thermodynamic and engineering computations, particularly when examining a fuel’s total energy content. When taking into account the energy released in equipment like boilers and furnaces—where the water vapor is not condensed but instead continues in the gaseous state—LCV becomes more important.
FAQs
- What is the purpose of Higher Calorific Value (HCV) and Lower Calorific Value (LCV)?
- HCV and LCV quantify the energy content of fuels when burned, which helps in energy system design and efficiency calculations.
- What is the key difference between HCV and LCV?
- HCV includes heat released from water vapor condensation, while LCV does not.
- Why does HCV provide higher values than LCV?
- HCV accounts for the latent heat of vaporization of water, which LCV excludes it.
- In which applications is HCV commonly used?
- HCV is often used in engineering and thermodynamic calculations.
- When is LCV more relevant in practical applications?
- LCV is more relevant when dealing with appliances where water vapor remains in the gaseous state, such as boilers and furnaces.
- Why is understanding these values important for energy consumers?
- Understanding HCV and LCV helps consumers make informed decisions about fuel selection and energy-efficient appliances.
- How can these values contribute to sustainability?
- By choosing the right fuel based on HCV or LCV, consumers can reduce energy waste and improve sustainability.
- Are HCV and LCV values universal for all fuels?
- No, these values vary from one fuel type to another due to the composition of fuels.
- What units are used to express HCV and LCV?
- They are typically expressed in units such as joules per kilogram (J/kg) or British thermal units per pound (BTU/lb).
- Can you give an example of a situation where knowing HCV and LCV is crucial?
- When selecting a fuel for a power plant, understanding HCV and LCV helps in maximizing energy output and efficiency while minimizing environmental impact


Very nice, thank you :))
Thank you sir. The article is very useful .
nice
superb…..
Yaa
Exactly
Thanks a lot
why it is not possible to condense water vapour in LCV
The water formed during the combustion if not allowed to condense, it will not release its latent energy. This is the reason, LCV does not contain the Latent heat of water term in it.
Nice discretion
[…] needs to use and select fuel based on its properties like calorific value, density, viscosity, specific heat, and flash point, and fire point. Now, we will understand first […]