Why should we study the ideal Carnot cycle if it is not practically possible? This is an obvious question in every mechanical engineer’s mind while studying thermodynamics or refrigeration and air conditioning topics. But Why? Here is the answer:
We as human beings make machines to ease our work by utilizing the concepts of nature. Mankind always wants some kind of machine to help them work better and more efficiently. The tasks could be repetitive in nature and hence they need to run the machine as long as they want the work to be delivered.
Different processes make a cycle, and the repetition of these cycle by repeating processes again and again keep the machine running. In the real world, the processes are always real and with losses like friction, etc. The Carnot cycle is an ideal cycle that can be considered a benchmark cycle giving maximum efficiency in similar conditions.
Table of Contents
Ideal Carnot Cycle
The ideal Carnot cycle is a thermodynamics cycle that consists of four different reversible processes. These processes are
- isothermal compression,
- adiabatic compression,
- isothermal expansion, and
- adiabatic expansion.
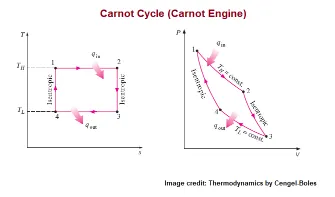
All four processes are reversible in nature with no irreversible losses. The losses that cannot be reversed are losses like friction or local heat transfer. The losses are undesirable and it reduces the workout from the given machine, cycle or engine. Thus, lower losses produce more work output from the given heat input in the cycle or engine.
If someone claims that he or she constructed or devised a real engine giving more efficiency than the Carnot cycle/engine working between the same temperature limits, the claim is false. The real engine always generates irreversible losses and reduces the power output and hence lower efficiency.
For example, temperature limits of 300 K and 500 K give ideal efficiency of 40 %. This is the upper limit of efficiency of all real engines working between these temperature limits. So, if a person claims that his real engine works between 300 K and 500 K having an efficiency of more than 40 % (greater than Carnot efficiency), this claim is not acceptable.
The same theory can be applied to refrigerators also. The Carnot refrigerator gives a maximum limit of COP (Coefficient of Performance) for any actual refrigerator working between those temperature limits.
The ideal Carnot cycle or engine efficiency or Carnot refrigerator COP does not depend on the working substance (fluid flowing in the machine). Thus, the fluid properties do not have any effect on the performance of an ideal engine or refrigerator but in real life, the different working substance produces different work output.
Conclusion
Thus, the Carnot engine or cycle is the benchmark for all real engines or refrigerators and gives the upper limit of efficiency and coefficient of performance. It helps us to understand the basic underlying principles of thermodynamics regarding heat engines and heat pumps. It also serves as the base of efficiency and coefficient of performance comparison benchmark. It inspires the design engineer to design a given heat engine efficiently and optimize it to get the maximum possible output.
FAQs on Carnot Cycle
Why is the ideal Carnot cycle a benchmark for heat engines in theory?
As a benchmark for actual engines, the ideal Carnot cycle indicates the highest efficiency achievable for a heat engine running between two temperature reservoirs.
What distinguishes the main elements of the Carnot cycle from those found in actual engines?
There are two isothermal processes and two adiabatic processes in the Carnot cycle. Since it functions reversibly and without losses, which is practically impossible, it varies from real engines.
How does the temperature difference between the hot and cold reservoirs affect the Carnot efficiency?
The only factor affecting the Carnot efficiency is the reservoir temperatures. High-temperature heat sources are essential because efficiency rises as the temperature difference between them widens.
Despite the fact that it is not practically possible, why is it important to study the Carnot cycle?
The Carnot cycle provides insight into the thermodynamic principles that govern real engine design and development, as well as the top limit of efficiency for heat engines.
Can modern engines match the Carnot cycle’s efficiency?
The answer is that irreversibilities like friction, heat loss, and poor heat exchange prevent real-world engines from operating at Carnot efficiency. Engineers work to improve design and operations in order to come as close as possible.

[…] Compression Refrigeration System/plant works on the principle of a reversed Carnot cycle. Normally, any refrigeration system consists of four main types of components: 1. Compressor 2. […]